The Why: Mosquito-borne viruses are a growing global health threat, driven by climate change, population growth, and global travel. Despite advances in vaccines and treatments, preventing epidemics remains a major challenge.
The How: At the Noval Lab, we use cutting-edge in vitro and in vivo models to study emerging alphaviruses such as chikungunya (CHIKV) and Mayaro (MAYV).
The What: We seek to uncover how these viruses cause severe and long-term disease, with the ultimate goal of translating this knowledge into strategies that reduce the burden of future epidemics.
-
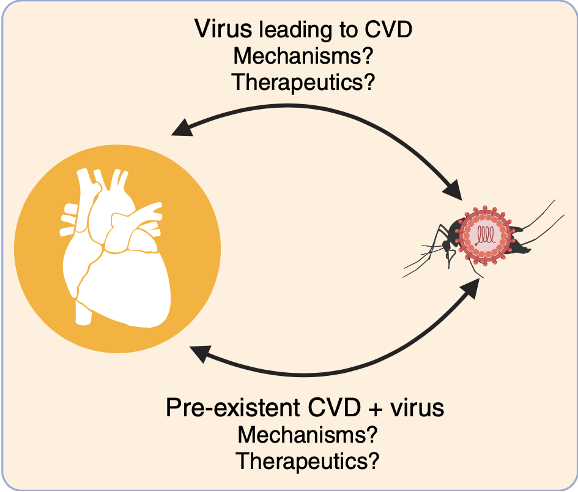
Emerging viruses can disrupt cardiovascular health, as seen during the COVID-19 pandemic. Mosquito-borne viruses such as chikungunya (CHIKV), dengue, and Zika have been linked to arrhythmias, myocarditis, heart failure, and even sudden death. Although the heart is not a primary infection site for these viruses, viral RNA and proteins have been detected in cardiovascular tissues, suggesting direct effects on these organs.
At the Noval Lab, we investigate how alphaviruses like CHIKV infect the heart and vasculature, focusing on viral persistence, immune responses, and chronic inflammation. Our work shows that inefficient viral clearance can lead to long-term vascular damage, raising concern for persistent CVD after infection (Noval et al., Nat. Commun., 2023).
We aim to uncover how mosquito-borne viruses like CHIKV disrupt cardiovascular health, knowledge that will not only reveal fundamental virus–host interactions but also inform strategies to prevent long-term complications in vulnerable populations.
-
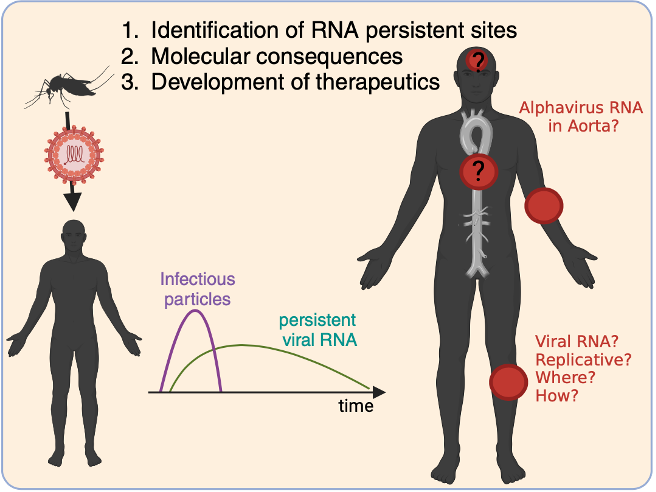
Acute RNA viruses were once thought to be fully cleared after infection, but sensitive detection methods have revealed that viral RNA can persist in cellular reservoirs. For alphaviruses such as CHIKV, SINV, and VEEV, this persistence is linked to chronic inflammation, post-acute sequelae, and long-term complications.
At the Noval Lab, we investigate where viral RNA hides, how it persists, and its impact on disease. Using advanced infection models and single-cell technologies, we aim to define the viral and host factors that allow persistence and determine whether persistent RNA can generate new infectious virus.
Key Questions We Ask:
Where does viral RNA persist, and in which cell types?
What viral and host factors promote or restrict persistence?
Is persistent RNA full-length, and can it reactivate?
Our ultimate goal is to develop strategies to eliminate persistently infected cells, preventing chronic complications and reshaping how we think about acute RNA virus infections.
-
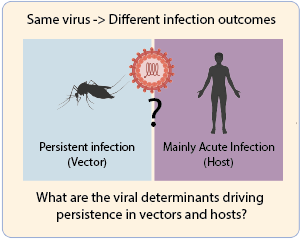
Alphaviruses can establish persistent infections in their vectors, while the same viral genome is capable of causing a completely lytic infection in vertebrates. This dual behavior raises important questions about how viral and host factors shape the outcome of infection.
At the Noval Lab, we aim to develop models of persistence in mosquito cell lines and live vectors to uncover the viral determinants of persistence specific to invertebrates, vertebrates, and those shared across both hosts.
By dissecting these processes, we aim to uncover the fundamental principles that govern persistence versus clearance, offering new insights into the biology of alphaviruses and the complexity of virus-host interactions.